New Drug Lucentis Restores Eye Sight for Wet Age-Related Macular Degeneration
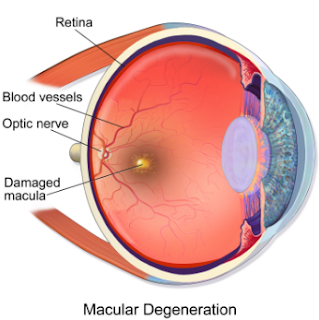
Genentech’s new drug Lucentis is the first proven treatment to restore sight in a number of patients with the “wet” form of age-related macular degeneration (AMD). This disease affecting nearly 1.2 million Americans over age 40 is the major cause of blindness in the elderly. Leaking of blood vessels in the back of the eye invade the retina and erode vision. Thus, the term “wet” is used for this particular form of sight deterioration.
Other treatments have been shown to slow the rate of vision loss, but no other drug in clinical trials has proved to restore or improve a portion of eyesight. Lucentis helped 95% of clinical trials subjects to preserve the sight they had remaining. One-third of patients in clinical trials were able to read three more lines on an eye chart after treatment with Lucentis. For those already experiencing major vision loss, the new drug will be too late to be of benefit.
Two drugs, Macugen and Visudyne, produced by other companies are currently used to slow or prevent further vision loss. The biggest competing drug, Avastin, is also produced by Genentech, but it was primarily designed to treat colon cancer. Its “off-label” use has been effective in treating some patients with AMD. However, Avastin has not been through the rigors of clinical trials as a treatment for sight loss. The company will not block its off-label use, but emphasizes that Lucentis is the preferred drug for treatment of this condition.
The major drawback for Lucentis is its high cost – about $1,950 per injection. To be effective, a patient will need five to seven injections in the first year of treatment. Avastin, at $50 a dose, is a bargain and for some patients may work almost as well.
Genentech has set up programs to provide Lucentis free to uninsured patients and to cover Medicare co-payments for qualified patients.
The injection in the eyes carries a risk of discomfort, inflammation and increased pressure. A topical drug may be developed to avoid these potential side effects, making Lucentis even more appealing for use by doctors for their patients.
Lucentis will not completely restore vision or reverse the aging process, but for some, timely treatment may enable them to continue driving and living independently. Lucentis was recently approved for use by the FDA.
Other treatments have been shown to slow the rate of vision loss, but no other drug in clinical trials has proved to restore or improve a portion of eyesight. Lucentis helped 95% of clinical trials subjects to preserve the sight they had remaining. One-third of patients in clinical trials were able to read three more lines on an eye chart after treatment with Lucentis. For those already experiencing major vision loss, the new drug will be too late to be of benefit.
Two drugs, Macugen and Visudyne, produced by other companies are currently used to slow or prevent further vision loss. The biggest competing drug, Avastin, is also produced by Genentech, but it was primarily designed to treat colon cancer. Its “off-label” use has been effective in treating some patients with AMD. However, Avastin has not been through the rigors of clinical trials as a treatment for sight loss. The company will not block its off-label use, but emphasizes that Lucentis is the preferred drug for treatment of this condition.
The major drawback for Lucentis is its high cost – about $1,950 per injection. To be effective, a patient will need five to seven injections in the first year of treatment. Avastin, at $50 a dose, is a bargain and for some patients may work almost as well.
Genentech has set up programs to provide Lucentis free to uninsured patients and to cover Medicare co-payments for qualified patients.
The injection in the eyes carries a risk of discomfort, inflammation and increased pressure. A topical drug may be developed to avoid these potential side effects, making Lucentis even more appealing for use by doctors for their patients.
Lucentis will not completely restore vision or reverse the aging process, but for some, timely treatment may enable them to continue driving and living independently. Lucentis was recently approved for use by the FDA.



Comments
Post a Comment